Introduction:
In the world of healthcare, medical devices play a crucial role in diagnosis, treatment, and patient care. At the same time ensuring their proper identification, usage, and safety is of paramount importance. This is where medical device labelling steps in, providing essential information to healthcare professionals and patients alike. In Chapter VI of MDR 2017, The Ministry of Health and Family Welfare (MoHFW) of India has outlined the labelling requirements for medical devices, underlining the significance of clear and informative labelling in the healthcare industry. These regulations, integrated within the larger framework of the Medical Devices Rules, 2017, go beyond being a mere legal obligation. They are a strategic approach towards enhancing patient safety, ensuring proper usage, and facilitating effective healthcare delivery.
Importance of Medical Device Labelling:
Medical device labelling serves as a bridge of communication between manufacturers, healthcare providers, and patients. It goes beyond branding to convey critical information about the device, its purpose, usage instructions, and manufacturer details. Accurate and comprehensive labelling ensures the proper identification, handling, and administration of medical devices, thereby contributing to patient safety and effective healthcare delivery.
Key Labelling Requirements:
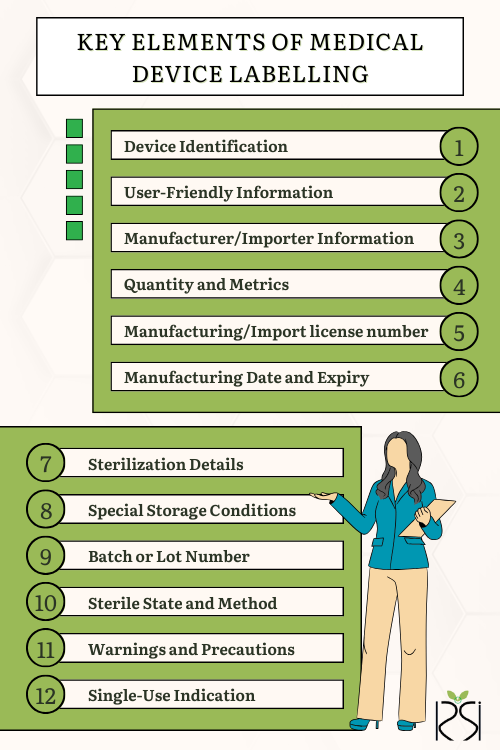
According to the Medical Device Rule 2017, specific details must be included on the labels of medical devices:
- Device Identification: The device's name, batch number, or serial number for identification.
- User-Friendly Information: Essential details for users to identify the device and understand its use.
- Manufacturer/Importer Information: Manufacturer's/Importer’s name, address, and manufacturing premises.
- Quantity and Metrics: Correct statement of net quantity, expressed in weight, measure, volume, or units.
- Manufacturing/Import license number: The manufacturing/import license number for all locally manufactured/imported medical devices.
- Manufacturing Date and Expiry: Month and year of manufacture, and expiry or shelf life information.
- Medicinal or biological substance: If applicable, label should indicate that the device contains medicinal or biological substance.
- Sterilization Details: For sterile devices, the date of sterilization or recommended shelf life.
- Free sample for medical professional: If the device is intended for distribution to medical professionals as a free sample then the label must be overprinted with the words “Physician’s Sample- Not to be sold”.
- Special Storage Conditions: Indications of any required storage or handling conditions.
- Batch or Lot Number: A distinctive batch or lot number for traceability.
- Sterile State and Method: Indication of the device's sterile state and sterilization method.
- Warnings and Precautions: Relevant warnings or precautions for users' attention.
- Single-Use Indication: Labelling for devices intended for single use.
Labelling for Evaluation and Clinical Investigations:
Medical devices intended for clinical investigation, evaluation, testing, or training purposes must also be appropriately labelled. This includes details such as the product's name, batch or lot number, manufacturing date, use-before date, storage conditions, manufacturer's information, and the purpose for which it was manufactured.
The Legal Metrology (Packaged Commodities) Rules 2011
In addition to the labelling requirements as mentioned in the MDR 2017, the label must also meet the requirements as specified in the “Legal Metrology (Packaged Commodities) Rules, 2011” (LM rules) and amendments thereof. Related FAQ’s for this act can be found here.
Conclusion:
Medical device labelling is more than just printing information on a package – it's a cornerstone of patient safety, healthcare efficacy, and regulatory compliance. Accurate and comprehensive labelling empowers healthcare professionals to make informed decisions, enhances patient trust, and contributes to the seamless functioning of the healthcare ecosystem. As the landscape of medical devices continues to evolve, robust labelling practices remain at the forefront of ensuring the highest standards of care and safety. Medical Device labels must comply to requirements as prescribed in the MDR 2017 and LM rules (and their amendments thereof).
Consult Regulatory Solutions India for Your Labelling Compliance:
With over 12 years of invaluable experience in the regulatory domain, Regulatory Solutions India stands as a trusted partner. Our track record speaks volumes, with over 450 medical products successfully registered. What's more, we've garnered satisfied clients from more than 15 countries, underscoring our global reach and impact.
For all your regulatory needs, including labelling compliance, Regulatory Solutions India is your go-to resource. Contact us today to elevate the effectiveness of your healthcare endeavors. Your success is our commitment.
FAQ’s
1. What is the role of medical device labelling in healthcare?
Medical device labelling serves as a vital means of communication between manufacturers, healthcare providers, and patients. It provides essential information about the device's purpose, usage instructions, and manufacturer details, contributing to patient safety and effective healthcare delivery.
2. What are the key requirements for medical device labelling?
According to the Medical Device Rule 2017, medical device labels must include details such as device identification, manufacturer information, quantity and metrics, manufacturing date and expiry, sterilization details, storage conditions, warnings, batch or lot number, sterile state indication, and more. In addition, medical device labels must comply to LM Rules requirements.
