The Central Government has launched the National Single Window System (NSWS) portal for businesses/investors seeking to enter or operate in the Indian market . It serves as a single window where more than 600 central and 6000 state approvals and clearances. The objective behind launching this online NSWS portal is to provide an efficient and transparent system for advising emerging and existing business on applicable clearances and approval.
In this blog we provide a quick guide to the process flow for submitting applications on this portal and also the CDSCO approvals currently incorporated therein for medical devices.
Using the NSWS Portal: Your Easy Guide to Approvals
Getting Started: Any business can create an account on the NSWS portal by providing basic company information to create a profile. The portal features a handy tool called “Know Your Approvals'' which asks questions about planned business activities and then identifies the regulatory approvals likely required. You can add these approvals to your dashboard for easy tracking.
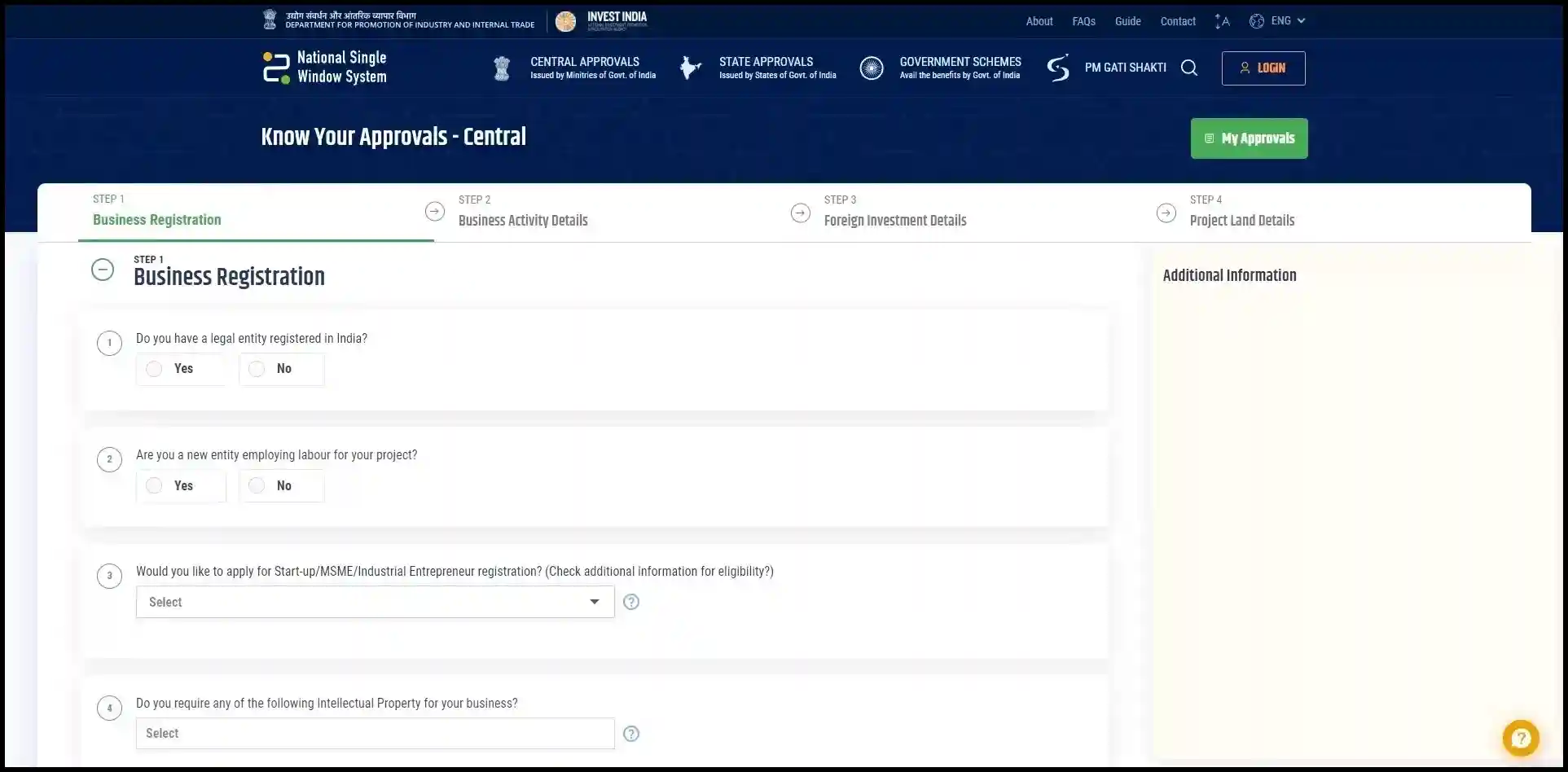
Applying for Approvals: The portal offers straightforward digital application forms for various central government approvals. Mandatory fields are indicated in the forms, and some information may auto-populate from your profile. Helpful tooltips explain what information is needed in each field. Progress can be saved, allowing for completion of applications at a later time.
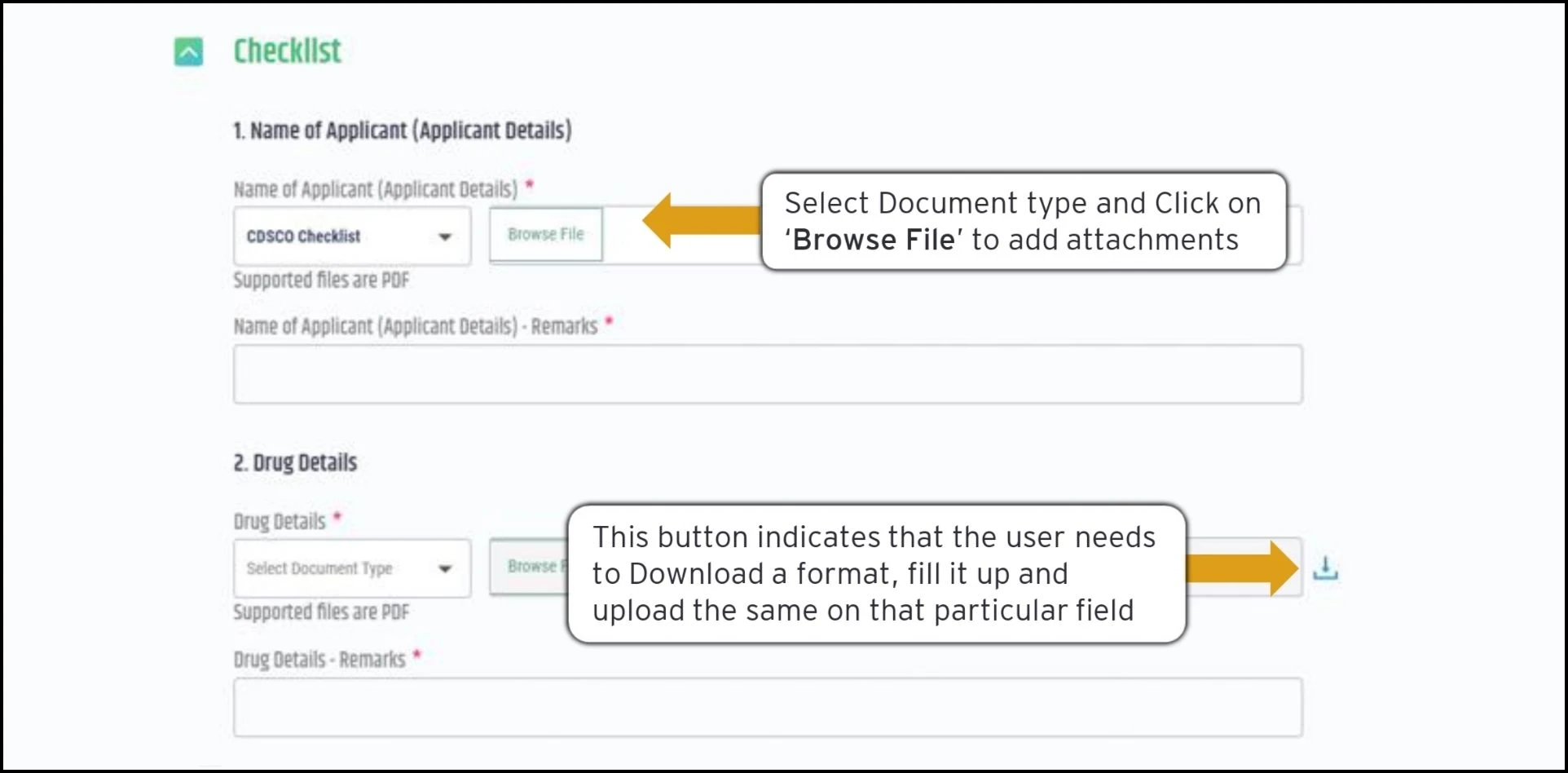
Uploading Documents: Relevant checklists assist in identifying the documents needed to support each application. Users simply browse their computer and upload files directly to the portal. Standardized formats are available for download if certain documents must follow a template.
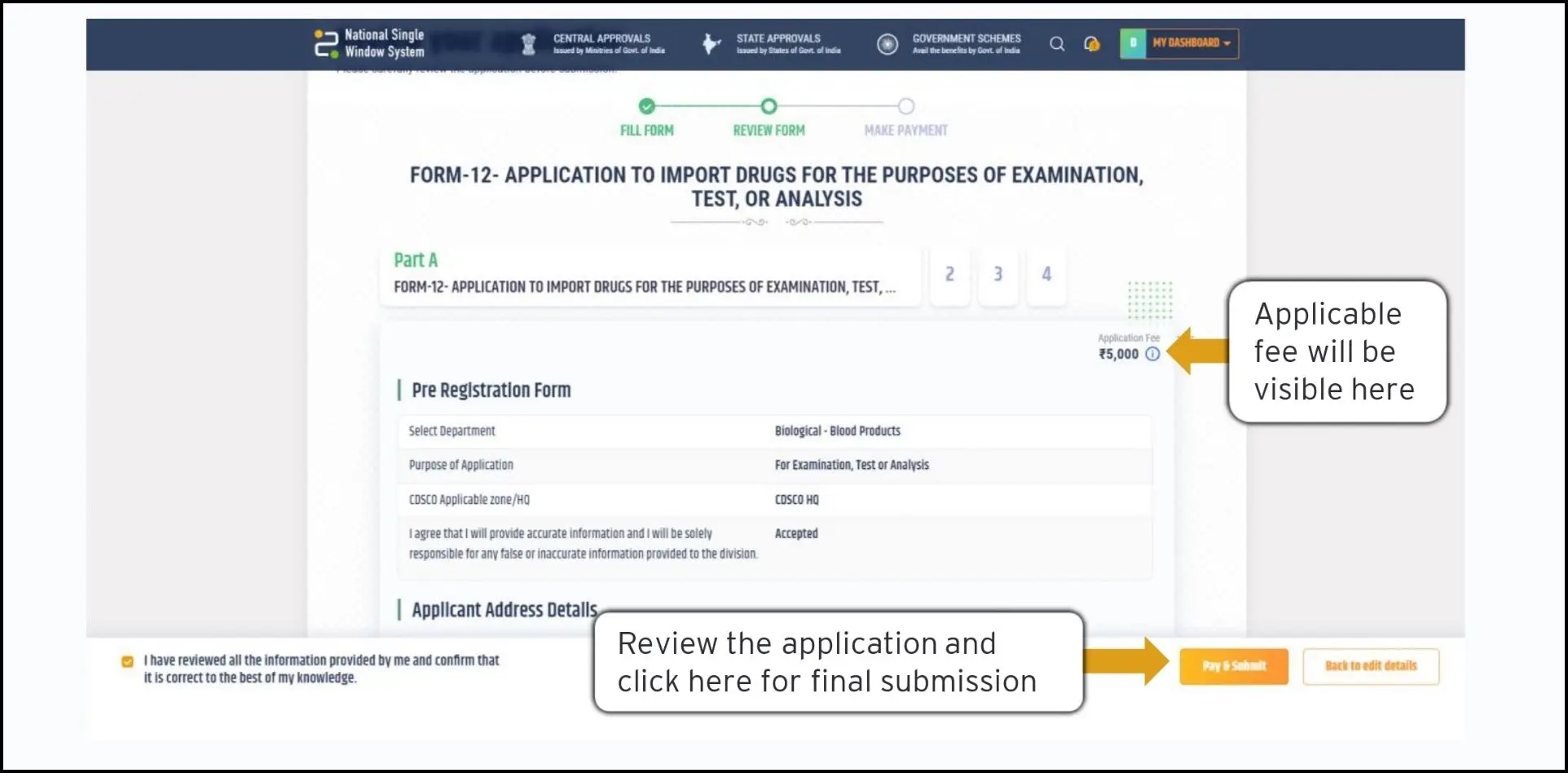
Final Review & Fees payment: Upon completion, the portal allows users to review the full application and make edits. The applicable fee is also indicated at this stage and the applicant can proceed to pay the same.
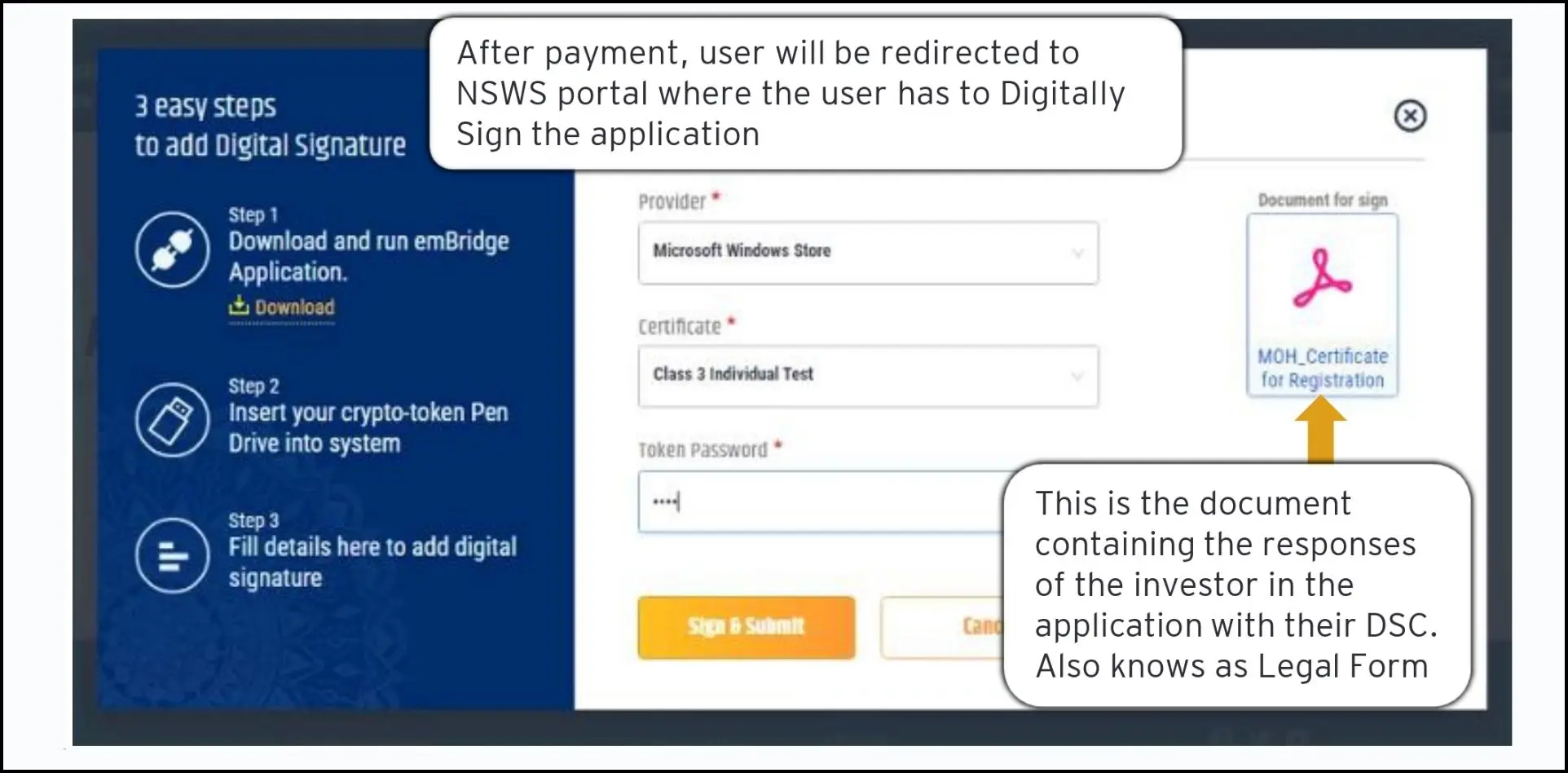
Application Submission: The final legal form is digitally signed using a Digital Signature Certificate (DSC) and submitted to the government department for processing and approval.
Tracking Applications: Post the application submission the portal provides a running status update on all applications, allowing users to monitor their progress. Users also have the option to submit additional documents if requested by the reviewing authority.
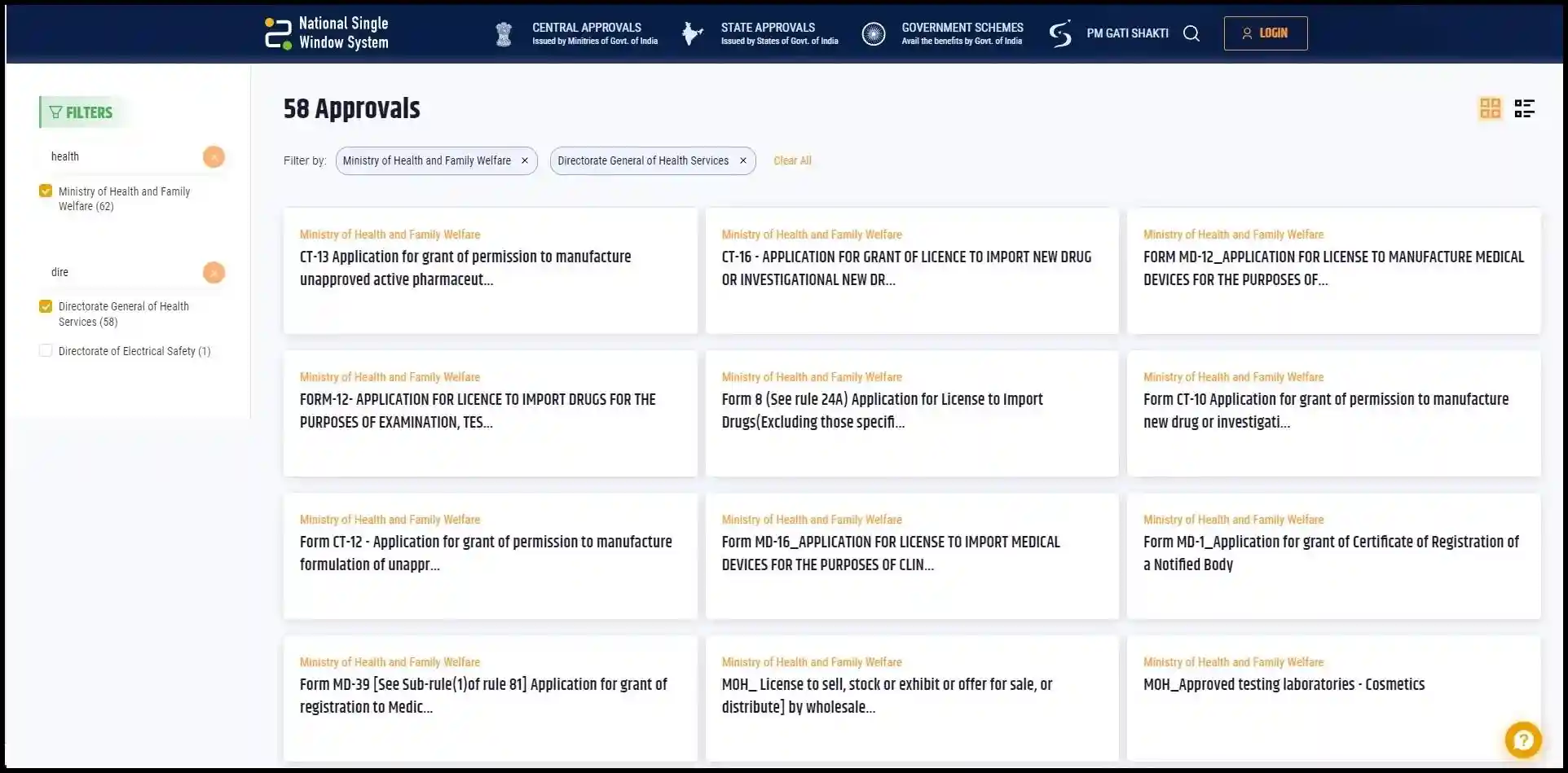
Services Available on the NSWS Portal for Medical Devices Industry:
Currently, the NSWS portal provides the following approvals/registrations for the medical device industry:
- Form MD-1 Application for grant of certificate of registration of a notified body.
- Form MD-12 Application for license to manufacture medical devices for the purposes of clinical investigations or test or evaluation or demonstration or training.
- Form MD-16 Application for license to import medical devices for the purposes of clinical investigations or test or evaluation or demonstration or training.
- Form MD-39 Application for grant of registration to medical device testing laboratory for carry out test or evaluation of a medical device on behalf of manufacturer.
As the NSWS portal is in an early stage, CDSCO is constantly adding more approval and registration services to the portal. It is recommended to keep an eye on the official NSWS portal for updated services.
Benefits of the NSWS Portal:
The NSWS portal offers several benefits including:
- Simplifying and centralizing the identification of required approvals through an intuitive approval finder based on business details.
- Providing clear application forms and checklists outlining exactly what submission documents are mandated by central / state agencies (including CDSCO).
- Allowing fully digital application submission, including online payments, rather than physical paperwork submissions.
- Enabling real-time tracking of application status through online dashboards for better consultant support.
Conclusion:
The NSWS portal aims to streamline and bring consistency to India's business approval process through a single unified platform. Its online tools help demystify regulatory requirements, making it easier for both Indian and foreign companies to invest in and operate within the country.
About Regulatory Solutions India (RSI):
With over 12 years of expertise in successfully navigating the regulatory landscape of healthcare product registration in India, Regulatory Solutions India (RSI) stands as a reliable partner. RSI specialises in hand holding clients along the complete regulatory approval process cycle, ensuring compliance, and supporting businesses in bringing their medical innovations to the dynamic Indian healthcare market.
Contact us for all your regulatory needs.
Image Source – Official CDSCO Notification
FAQs:
What is the NSWS portal, and how does it simplify the medical device approval process in India?
The NSWS portal is a Single Window System launched by the central government which acts as a one-stop shop for all approvals required by investors and facilitates ease of doing business.
Can any business easily create an account on the NSWS portal, and what information is required?
Yes, any business can create an account by providing basic company information to create profile on the NSWS portal.
How does the "Know Your Approvals" tool assist businesses in obtaining regulatory clearances?
The tool asks questions about planned business activities and identifies the approvals required, providing a clear roadmap for businesses.
What services and approvals are currently available on the NSWS portal for the medical device industry?
The NSWS portal currently offers various central and state related approvals. For the most up-to-date information and new services, businesses are recommended to regularly visit the official NSWS portal.
What are the key benefits of using the NSWS portal for medical device approvals in India?
The benefits include simplified identification of required approvals, clear application forms, fully digital submission, and real-time tracking of application status, making the process efficient and user-friendly.
